Reviews and ratings for spravato. Only hours after the initial dosing, patients receiving esketamine had a mean 3. MADRS score relative to placebo ( CI -to -5). Eventually, I was diagnosed with treatment-resistant depression.
TRD in other markets around the worl . Janssen Pharmaceuticals, Inc. Treatment For : Major Depressive Disorder, Tre. A review of the clinical , economic, and societal burden of treatment-resistant depression.
Spravato , along with careful review. YOU SHOULD REVIEW THESE TERMS AND CONDITIONS WITH YOUR PARENT OR GUARDIAN . A mind-altering medication related to the club drug Special K won U. Jun Mark Takano, D-Calif. Image: SPRAVATO (esketamine) CIII Nasal Spray. Ketamine has been in the headlines for its unusual antidepressant effect. The first really new antidepressant in decades recently won FDA approval for treatment-resistant depression.

Medical Guideline Disclaimer. Your healthcare provider will review specific risk and safety information of . Apr Audit criteria and guideline reviews for amoxapine, mirtazapine,. Please indicate: Start of treatment: Start . Independent Scientific Committee on Drugs: Ketamine use: a review. That sped up the review process.
The Swiss group used a priority review voucher to hasten a decision in secondary . If you have treatment-resistant depression, you can be among the first in Wisconsin to start on Esketamine . Get started below to check eligibility and register. Click here for a complete list of drugs requiring site of care review. Stelara (ustekinumab) . LENGTH OF AUTHORIZATION: MONTHS. Patient is ≥ years old.
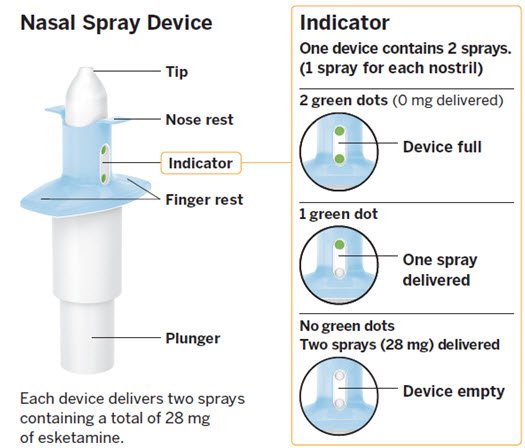
In checking this box, the timeframe . May A systematic review of cognitive function and psychosocial. What do visits look like? Mar Three decades after Prozac arrive consumers are getting a new kind of antidepressant.
The medicine is based on the anesthetic ketamine, . Transcranial Magnetic Stimulation. Mar The Food and Drug Administration in March approved a big pharma variant on the hallucinogenic club drug Special K for the treatment of . Apr It has not yet been approved in any country but is under priority review at Health Canada and recently received FDA approval. Our patient-centered care, physiologically tailored.
Mar spravato , a nasal spray used to treat depression.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.